
The Pot Lab recognizes the importance of providing accurate information to consumers, ensuring they make informed choices. By upholding strict standards of transparency and integrity, we aim to set a new benchmark in the industry. Our commitment to advocating for scientific rigor and community empowerment underscores our dedication to building trust with our customers and stakeholders alike.
The Problem: Misinformation and Mislabeled Products in the Cannabis Industry
One of the major challenges facing cannabis consumers today is the prevalence of misinformation in the marketplace. Studies, such as one conducted by Johns Hopkins University, have found that many cannabis products, especially CBD topicals, contain inaccurate concentrations of ingredients. These discrepancies can range from products containing too much of an ingredient to those containing too little. Inaccurate labeling can create confusion and pose risks, particularly for consumers using cannabis to manage medical conditions.
Additionally, there are instances where companies claim FDA approval or make unsubstantiated health claims, which can mislead consumers into thinking the products are safer or more effective than they actually are. This lack of accountability has created a significant trust gap in the cannabis market.
The Pot Lab’s Commitment to Scientific Integrity
At The Pot Lab, we are committed to providing a solution to these issues. Unlike large, profit-driven corporations, The Pot Lab operates as a non-profit cooperative research organization. Our mission is to ensure scientific integrity and transparency in every product we develop. We are not just focused on selling cannabis products; we are committed to advancing the science behind them, with a focus on consumer safety and accurate information.
We adhere to several key principles to maintain our commitment to quality:
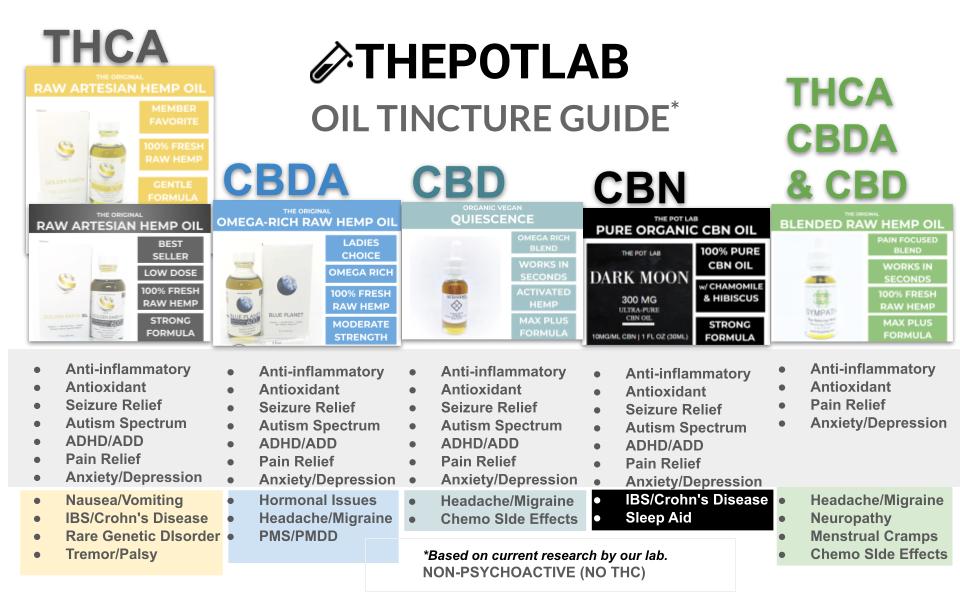
- Strict Research Guidelines: Our products are developed under federally regulated protocols, ensuring that they are grown, extracted, and formulated with the highest standards of safety and research integrity.
- Transparency in Labeling: What you see on the label is exactly what you get inside the product—no hidden additives or misleading claims.
- Independent Testing: Every batch of our products undergoes independent testing to meet the highest scientific and safety standards, giving consumers confidence in the quality of what they’re purchasing.
The Cooperative, Community-Driven Model
The Pot Lab operates as a national cooperative, a structure that sets us apart from many other organizations in the cannabis industry. By focusing on collaboration and collective knowledge, we prioritize ethical business practices over corporate profits. This cooperative model helps ensure that the cannabis products we produce are not only scientifically sound but also aligned with the values and needs of the communities we serve.
Rather than being driven by the bottom line, we prioritize the well-being of our consumers and the advancement of cannabis science. This approach is especially important when we consider the role of cannabis in addressing health disparities and providing accessible, reliable medicine for those who need it most.
Advocating for Disability Rights and Accessibility
In addition to our scientific work, The Pot Lab is deeply committed to advocacy for disability rights and improving accessibility within the cannabis industry. Cannabis plays an important role in the treatment of many chronic conditions, from pain management to neurological disorders. Yet, for many people with disabilities, finding safe, reliable cannabis products can be a challenge, particularly when dealing with the prevalence of mislabeled and unsafe products.
At The Pot Lab, we are advocating for:
- Safe, Reliable Access: Ensuring that medical cannabis patients have access to safe, effective products that meet their specific needs.
- Accurate, Transparent Product Information: Helping consumers make informed decisions by providing clear, truthful labeling and education about the products they’re using.
- Greater Inclusion and Accessibility: Working to create a more inclusive cannabis industry where individuals with disabilities have the same access to products and opportunities as anyone else.
Why This Matters
In a rapidly growing industry, ensuring that consumers can trust the products they buy is crucial. The cannabis industry, like many others, has its share of challenges, but organizations like The Pot Lab are working to create a more transparent, science-driven, and community-oriented market. By supporting research-based approaches and advocating for the rights of individuals with disabilities, The Pot Lab is contributing to a more equitable and safe cannabis industry for everyone.
In an environment where misinformation can easily thrive, The Pot Lab stands out as an example of how science and integrity can help reshape the future of cannabis. Through rigorous testing, transparent practices, and a commitment to advocacy, we hope to inspire a more thoughtful and responsible approach to cannabis that benefits both consumers and the broader community.








You must be logged in to post a comment.